Clinical Trials To Date
Synopsis
Amarasate® is a natural extract of New Zealand bitter hops that has been scientifically demonstrated to regulate eating behavior by reducing appetite, cravings, and caloric intake in just one hour. This ingredient and dosage format achieves this by specifically targeting the chemosensory enteroendocrine cells in the small intestine, promoting the release of naturally occurring hormones that suppress appetite (GLP-1 and CCK) from the enteroendocrine TAS2R receptors at six times the baseline levels, and acting on targeted brain regions to decrease appetite and gut mobility.
Clinical trials in both men and women have shown up to a 100% reduction in increased hunger and craving, as well as a 20% reduction in calorie intake.

Clinical 1
Amarasate Mode of Action
The Amarasate® extract was tested in a human clinical trial with 20 healthy men. The study involved a randomized, double-blind, placebo-controlled cross-over study (a study where neither participants nor researchers knew what capsules were taken, and each participant took each of the capsules one week apart). Participants’ food intake was measured after taking the Amarasate® extract delivered in a patented capsule that opened in the stomach or in the duodenum, or a placebo. The study showed that when Amarasate® was delivered to the duodenum, participants ate, on average, 944kJ (226 calories) less than with placebo over 3.5 hours.
This equates to an 18% drop in overall energy intake over that period.
An Extract of Hops (Humulus Lupulus L.) Moderates Gut Peptide Hormone Secretion and Reduces Energy Intake in Healthy-Weight Men: A Randomized, Cross Over Clinical Trial.
The American Journal of Clinical Nutrition 2022, 115(3), 925-940
Clinical 2
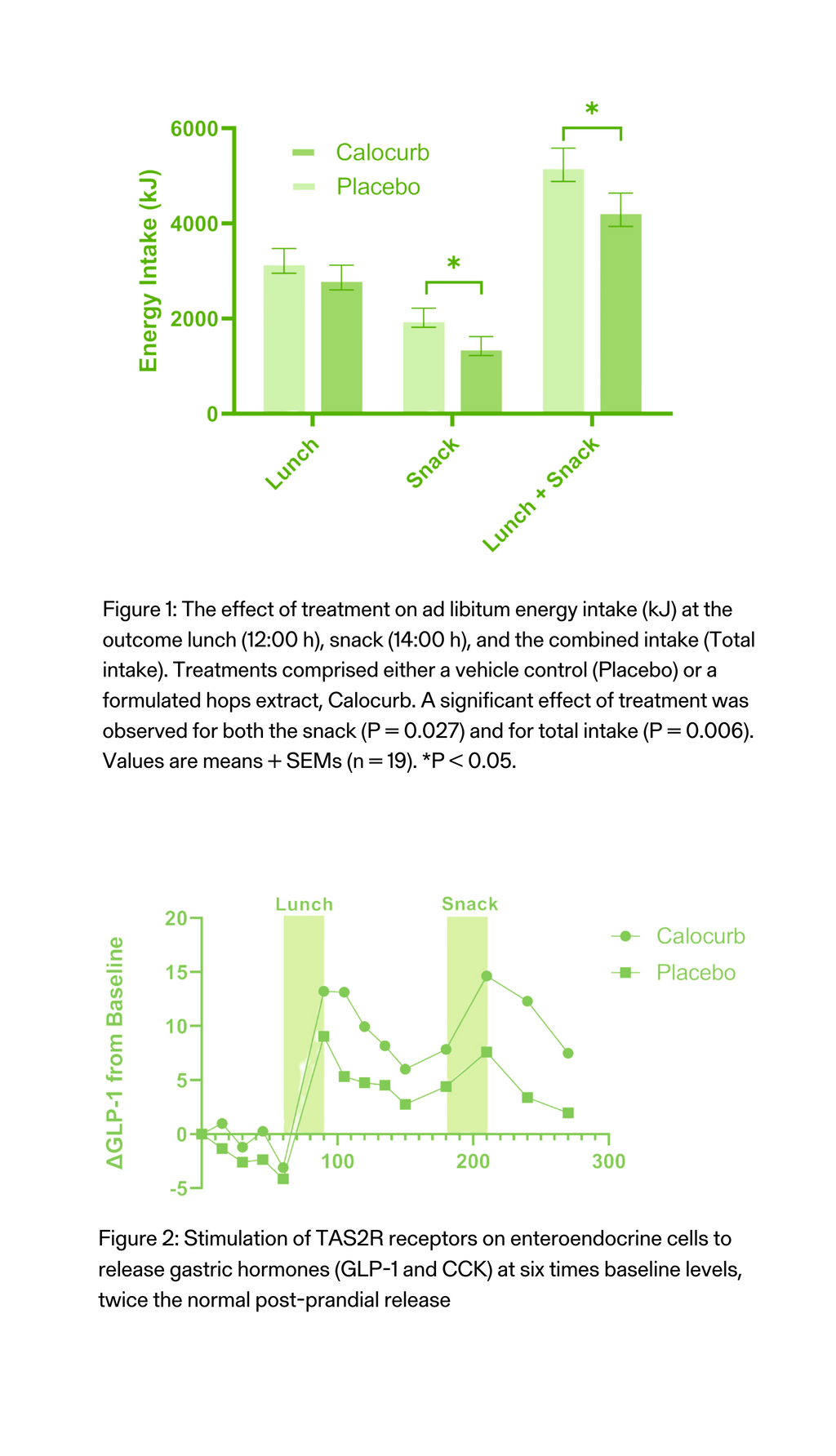
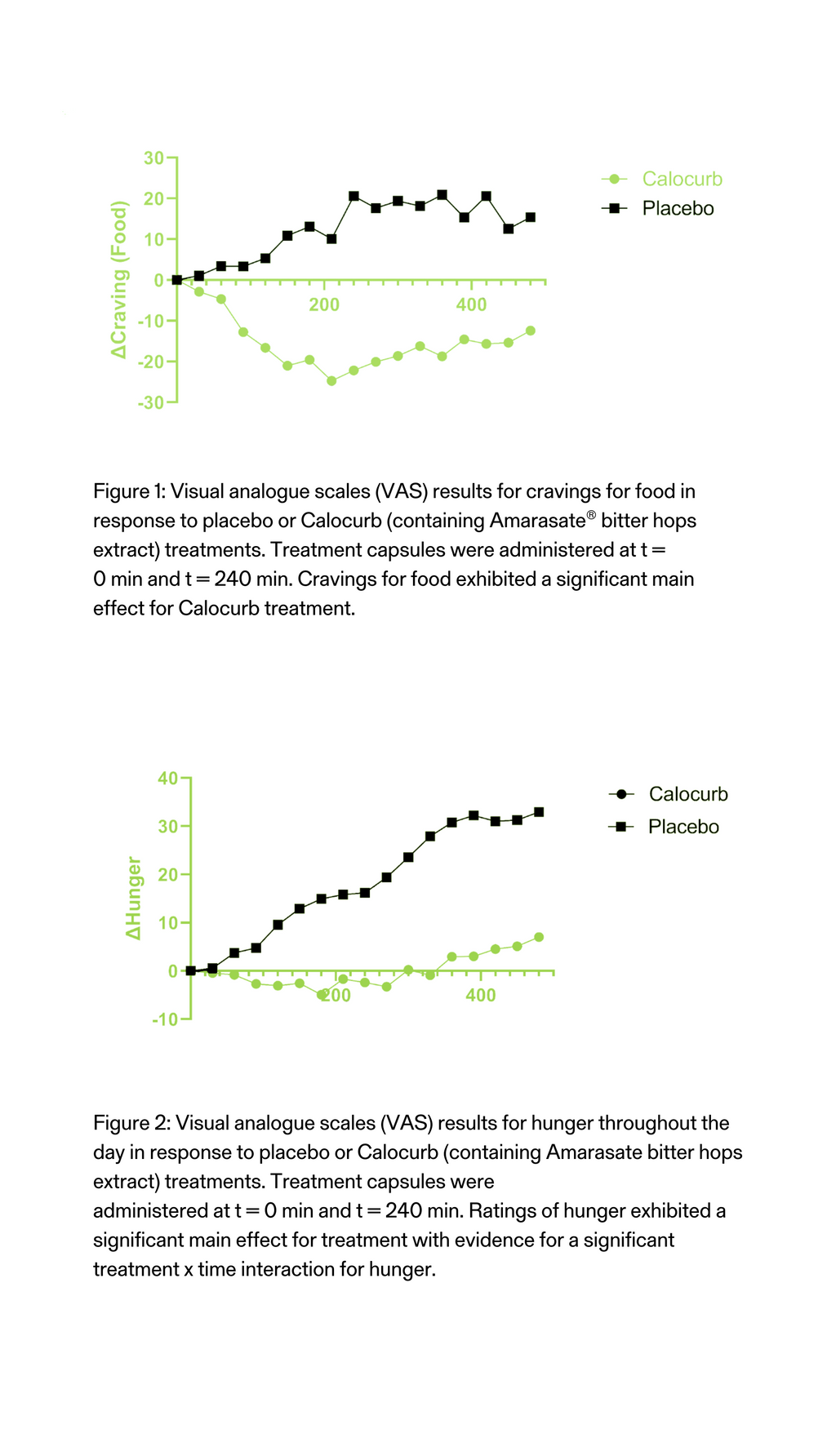
Hunger and Fullness in Men
The Amarasate®extract effect on appetite measures was tested in a human clinical trial with 30 healthy men. The study involved a randomised, double-blind, placebo-controlled cross-over study. Each participant took each of the capsules one week apart for three successive weeks.All treatment groups contained 2 capsules, one given at 16 h and the second given 20 h into the 24 h water-only fast, each containing half the total daily treatment dose. At 16 h into the 24 h fast (t= 0 min) participants recorded their baseline visual analogue scale (VAS)- based appetite assessments and then consumed a delayed digestion capsule containing either a placebo, a low-dose hops extract or a high-dose Amarasate® hops extract. Every 30 min, VAS-based appetite assessment questionnaires were taken from 16 h until 24 h into the 24 h fast. Then at 20 h into the 24 h fast (t= 240 min) a second treatment capsule match to the first was given.
- Participants experienced an 80% reduction in increased hunger.
- This equates to a 25% reduction in hunger overall.
Figure 1: Visual analogue scales (VAS) results for hunger throughout the day in response to placebo and Calocurb treatments. Changes in hunger ratings from the 16 h (t= 0 min) and 24 h (t = 480 min) period of the 24 h fast. Treatment capsules were administered at t = 0 min and t = 240 min. Hunger exhibited a significant main effect for the Calocurb treatment group with evidence for a significant treatment x time interaction for hunger.
Clinical 3
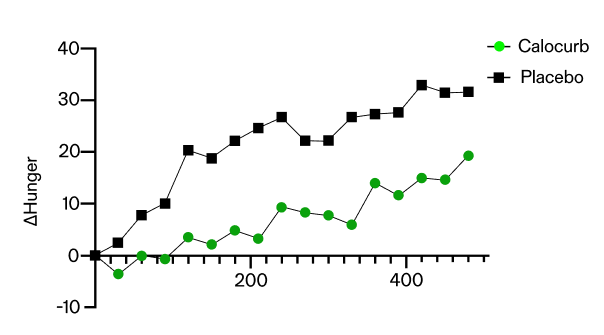
Hunger, Fullness and Craving in Women
The Amarasate® extract effect on subjective measures of appetite during the 16–24 h period of a 24 h water-only fast in 30 healthy, normal weight women. This study was modified from the previous study examining the effect of Amarasate® extract on appetite measures in men. This was a randomised, double-blind crossover treatment study. Each participant took each of the capsules one week apart for three successive weeks. The intervention arms were two formulation matched dosages of the Amarasate extract, high dose (high dose; HD, 500 mg total) and low dose (low dose; LD, 250 mg total), and a placebo treatment. At 16 h into the 24 h fast (t = 0 min), participants recorded their baseline Visual Analogue Scale (VAS) based appetite assessments and then consumed a delayed digestion capsule containing either a placebo, a low-dose hops extract, or a high- dose Amarasate® hops extract. Every 30 minutes, VAS based appetite assessment questionnaires were taken from 16 h until 24 h into the 24 h fast. Then, at 20 h into the 24 h fast (t = 240 min), a second treatment of capsules matching the first was given. This trial included an ad libitum rice-based meal at 24 h and VAS assessments related to appetite and food cravings, modifications from the original study design. Energy intake at the ad libitum meal was 14.3% lower when taking the HD treatment and 8.1% lower when taking the LD treatment relative to the placebo control treatment.
- Over the last 8 hours of a 24 hour fasting trial, females experienced a 100% reduction in change in hunger and a 120% reduction in change in food craving.
- This equates to a 30% reduction in hunger overall.
Upcoming Weight-Loss Trial
The Amarasate® extract effect on subjective measures of appetite during a 6 month weight-loss clinical trial in 150 men and women with a BMI >30. The study will compare the effect of Calocurb (Amarasate®) capsules taken twice daily to a matched placebo control on measures of weight loss, appetite/food craving, body composition, blood glucose and satiety hormones. Amarasate’s bioactivity has been validated in capsule form, showing potent stimulation of appetite suppressing gut hormones GLP-1 and CCK, and decreases in appetite and food intake.
These clinical results, combined with studies showing long-term weight loss from GLP-1 based therapies (both natural and synthetic mimics), indicates bioactivity favourable towards a weight loss application, and supports a likely positive outcome for the proposed clinical work.
Mode of Action
Calocurb's journey began with the discovery of Amarasate® in 2013, a groundbreaking, potent extract that has been demonstrated to stimulate the enhanced release of satiety hormones.Amarasate® is a natural extract of New Zealand bitter hops that has been demonstrated in clinical trials to regulate and reduce appetite, craving, and caloric intake. Amarasate ® is released into the small intestine, specifically the duodenum, from a delayed release patented capsule (Walker et al., 2022)\
- After only one hour, it stimulates the TAS2R receptors on enteroendocrine cells to release gastric hormones (GLP-1 and CCK) at six times baseline levels, twice the normal post-prandial release (Walker et al., 2022).
- These gastric hormones signal a part of the brain to decrease appetite and food cravings plus slow down the gastric emptying rate.
Quality and Safety
Calocurb is distributed in the US under the Dietary Supplement Health and Education Act (DSHEA) of 1994, regulated by the U.S. Food and Drug Administration. In the US the use of hops extract, or hops oleoresin, is approved by the U.S. FDA as an essential oil, oleoresin (solvent-free), and natural extract (including its distillate) which are generally recognized as safe (GRAS).
Amarasate®, of New Zealand bitter hops and the active ingredient in Calocurb is a naturally extracted used food-grade supercritical carbon dioxide (CO2) and conducted at New Zealand Hops, Inc (NZIPFR) facilities under current Good Manufacturing Practice (cGMP) guidelines. During this process, the levels of resins, tannins, polyphenols (8-PN), fats and waxes typically found in hops are reduced to negligible levels in the Amarasate® extract. The finished hops extract material is a thick, viscous, honey-like paste with an oily consistency, coloured golden to tan to greenish/brown, and exhibiting a typical hops aroma.
Audited stability testing is conducted on the bulk hops extract to ensure α-acid concentration is within standard level and this is tested periodically. Amarasate® is packaged in DR Licaps capsules at FDA, TGA, NFS and GMP-approved facilities at a Lonza company in Greenwood, SC, USA. Quality auditing and stability testing is diligently conducted every six months on new and existing batches including the bulk capsules and the finished packaged bottle.



